Peripheral Neuropathy: Chiropractor or a Pain Clinic Care?
July 22, 2025
Peripheral Neuropathy: Manage It Without Medication
August 25, 2025Knee discomfort slows life. Many desire non-surgical treatment. This page describes knee pain stem cell treatment, how it works, safety results, and who may benefit. Also covered is how a pain and neuropathy clinic could treat joint pain and nerve issues. This will help Arizona readers make confident, unambiguous next moves.
Know More About Knee Pain Stem Cell Therapy
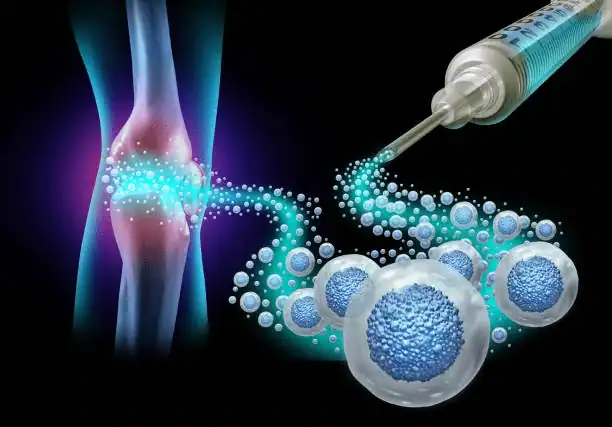
Knee stem cell treatment is a type of regenerative medicine that utilizes the patient’s stem cells to repair injured knee cartilage, reduce swelling, and stimulate the body’s natural production of growth factors.
Care professionals prepare patient cells and inject them into the knee under imaging surveillance and sterile conditions. Following their doctor’s orders, most patients go home the same day and resume modest activities.
How Does It Work?
Knee osteoarthritis stem cell injections may relieve pain and enhance function in the first 3–12 months, but long-term effects are unknown. A meta-analysis of clinical trials indicated considerable pain alleviation and better knee ratings compared to conventional alternatives, suggesting potential benefit in eligible individuals, though methods and cell sources vary. In some instances, symptoms improve without cartilage regeneration so that symptom reduction may be the sole near-term benefit.
Since studies employ varied cell sources, dosages, and schedules, specialists continue to explore optimal techniques and the longevity of outcomes.
Is Stem Cell Therapy Safe?
Autologous mesenchymal stem cell–based injections under sterile conditions show promising short-term safety. A 2024 comprehensive analysis of over 2,000 individuals revealed no short-term problems such as sepsis, infection, embolism, malignancy, hospitalization, or death. Most adverse events were injection site edema or soreness that disappeared within weeks. Knee injection side effects, including discomfort or swelling, are typically modest and short-lived, according to health information sources.
Safety depends on product type and processing, and long-term safety data is limited. Studies found greater transient reaction rates in specific sources. Working with skilled professionals who follow evidence-based guidelines and sterile procedures is crucial.
Key FDA and Policy Advice
In the US, FDA-unapproved stem cell products for orthopedic conditions such as knee osteoarthritis are available every day. The FDA advises patients to be aware of unlicensed cell product clinics and ask about regulations, dangers, and evidence. Insurers may consider mesenchymal stem cell treatment experimental for orthopedic applications, affecting coverage. The therapy may assist, but patients should discuss evidence, regulation, and informed consent before proceeding.
What About Nerve Discomfort and Neuropathy?
If your knees hurt, your feet or legs may tingle, burn, or become numb. When researchers look at pain and neuropathy, they often examine how circulation, nerve function, and musculoskeletal alignment affect joint pain and nerve symptoms. A pain and neuropathy clinic could discuss the pros and cons of stem cell treatment for neuropathy, particularly if nerve illnesses make it hard to accomplish everyday tasks. Metabolic support, targeted rehab, bracing, and other therapies may also be employed for joint treatment.
Expectations Both During and After the Course of Treatment
- Evaluation: During the evaluation process, the physician examines the knee, reviews the patient’s medical history, and may request imaging tests to determine whether the patient is suffering from arthritis or cartilage loss.
- Process: Most outpatient therapies consist of preparing sterile cells and injecting them into the knee.
- Recovery: Most patients resume mild activity shortly after, following a joint protection and mobility regimen. Short-term discomfort or swelling is likely.
- Follow-up: Regular rehab plans and check-ins monitor pain, swelling, strength, and function to advise return to sport or heavier activity.
What’s the Difference Between It and Surgery?
Hospitalization, extended recovery, and surgical risks are associated with severe arthritic knee replacement. Non-surgical stem cell therapy offers a faster recovery and fewer short-term side effects when done correctly, but long-term proof is limited. Many consider employing injections, treatment, and lifestyle changes to decrease discomfort now and delay surgery until necessary.
Clinic Questions You Should Ask
- What data supports your knee pain stem cell treatment approach, and what results do you track?
- Does the product have FDA approval or regulation, and what does it signify for risk and informed consent?
- What cell source, processing, and sterility/safety measures are used?
- What is the whole treatment plan (rehab, bracing, activity modifications) and how will progress be measured?
- Given that many insurers consider these treatments experimental, what are the overall expenses and coverage options?
The Bottom Line
With a thorough joint health plan, stem cell treatment for knee pain may relieve pain and improve function for some people, deferring surgery. Short-term safety with autologous techniques under proper conditions is shown, with most side effects being temporary pain or edema. Most orthopedic stem cell therapies are FDA-unapproved, insurers call them experimental, and long-term consequences are still being explored, so rigorous screening, informed consent, and skilled doctors are needed. For consultation, visit Restore Wellness Center.
Ready to Explore Stem Cell Therapy for Knee Pain?
Contact Restore Wellness Center today to discuss whether stem cell therapy is the right choice for your knee pain or neuropathy concerns. Schedule your consultation now.